Blueprint Medicines Corporation BPMC announced a definitive agreement to acquire San Diego, CA-based privately held biopharmaceutical company, Lengo Therapeutics, for $250 million in cash. The company will also make up to $215 million as potential payments based on the achievement of certain milestones.
The transaction is expected to close later in the fourth quarter of 2021 and is subject to customary closing conditions, including the expiration of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act.
Shares of Blueprint Medicines have declined 12.4% so far this year compared with the industry’s decrease of 15.8%.
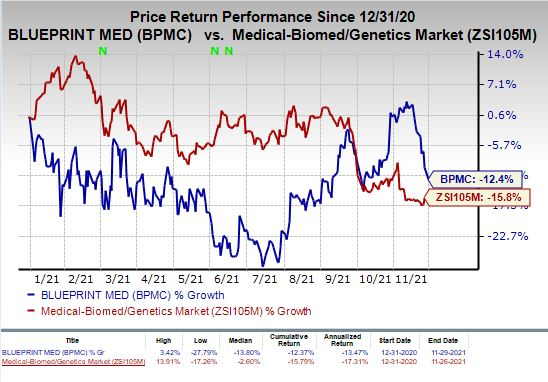 Image Source: Zacks Investment Research
Image Source: Zacks Investment Research
With the above-mentioned acquisition, Blueprint Medicines is looking to expand its precision oncology therapies and transform treatment for patients with EGFR-driven lung cancer. The acquisition will add Lengo Therapeutics’ lead compound, LNG-451, to Blueprint Medicines’ portfolio.
LNG-451, a potential best-in-class oral precision therapy, is currently being developed for the treatment of non-small cell lung cancer (“NSCLC”) in patients with EGFR exon 20 insertion mutations.
Data from pre-clinical studies have demonstrated that LNG-451 potently inhibits all common EGFR exon 20 insertion variants with marked selectivity over wild-type EGFR and off-target kinases. Also, LNG-451 is highly brain-penetrant and has shown compelling activity in a preclinical intracranial disease model.
Lengo Therapeutics plans to file an investigational new drug application to the FDA to begin clinical studies on LNG-451 in December 2021.
The acquisition of Lengo Therapeutics will also add additional undisclosed preclinical precision oncology programs to Blueprint Medicines’ portfolio for future drug discovery efforts.
Please note that Blueprint Medicines has started a phase I/II SYMPHONY study on BLU-945 – a triple-mutant EGFR inhibitor – to address patients with treatment-resistant EGFR-driven NSCLC. The company also plans to initiate a phase I/II study on BLU-701 for treating patients with EGFR-driven NSCLC later in the fourth quarter of 2021.
Zacks Rank & Stocks to Consider
Blueprint Medicines currently carries a Zacks Rank #3 (Hold). Better-ranked stocks in the biotech sector include Sarepta Therapeutics, Inc. SRPT, vTv Therapeutics Inc. VTVT and Editas Medicine, Inc. EDIT, all carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Sarepta Therapeutics’ loss per share estimates have narrowed 31.3% for 2021 and 26% for 2022, over the past 60 days.
Earnings of Sarepta Therapeutics have surpassed estimates in two of the trailing four quarters, and missed the same on the other two occasions.
vTv Therapeutics’ loss per share estimates have narrowed 21.7% for 2021 and 2.9% for 2022, over the past 60 days.
vTv Therapeutics’ earnings have surpassed estimates in three of the trailing four quarters and missed the same on the other occasion.
Editas Medicine’s loss per share estimates have narrowed 11.2% for 2021 and 4.6% for 2022, over the past 60 days.
Editas Medicine’s earnings have surpassed estimates in two of the trailing four quarters and missed the same on the other two occasions.
Tech IPOs With Massive Profit Potential: Last years top IPOs surged as much as 299% within the first two months. With record amounts of cash flooding into IPOs and a record-setting stock market, this year could be even more lucrative.
See Zacks’ Hottest Tech IPOs Now >>
Click to get this free report
Sarepta Therapeutics, Inc. (SRPT): Free Stock Analysis Report
vTv Therapeutics Inc. (VTVT): Free Stock Analysis Report
Blueprint Medicines Corporation (BPMC): Free Stock Analysis Report
Editas Medicine, Inc. (EDIT): Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
The views and opinions expressed herein are the views and opinions of the author and do not necessarily reflect those of Nasdaq, Inc.
www.nasdaq.com