Regeneron Prescribed drugs, Inc. REGN introduced that its antibody cocktail, casirivimab and imdevimab, for the therapy of COVID-19 has been permitted in Japan. The cocktail, referred to as REGEN-COV in america, was granted full approval in Japan below the identify Ronapreve.
The antibody cocktail was granted a Particular Approval Pathway below article 14-Three of the Prescribed drugs and Medical Gadgets Act in Japan.
The approval was primarily based on constructive outcomes from a section III research in high-risk non-hospitalized sufferers, which confirmed that the antibody cocktail diminished the chance of hospitalization or loss of life by 70% together with outcomes from a section I research that examined the security, tolerability and pharmacokinetics in Japanese folks.
We word that Regeneron has collaborated with Roche RHHBY to extend the worldwide provide of the antibody cocktail. Per the settlement, Roche is primarily chargeable for the event and distribution of the product exterior america.
Final 12 months, Chugai obtained the event and unique commercialization rights in Japan from Roche. Chugai is working with the Japanese authorities to make sure an applicable and well timed provide of the antibody cocktail.
The FDA has granted an Emergency Use Authorization (EUA) to REGEN-COV to deal with mild-to-moderate COVID-19 in adults and pediatric sufferers (12 years of age and older weighing ≥40 g) with constructive outcomes of direct SARS-CoV-2 viral testing, and people at excessive danger of development to extreme COVID-19, together with hospitalization or loss of life.
The FDA just lately up to date the EUA for REGEN-COV, decreasing the dose to 1,200 mg (600 mg casirivimab and 600 mg imdevimab), which is half the dose initially licensed.
Regeneron is in discussions with the FDA to broaden the present EUA to different populations, together with prevention and hospitalized affected person settings.
The corporate’s shares have gained 21.2% within the 12 months to date in opposition to the trade’s decline of three%.
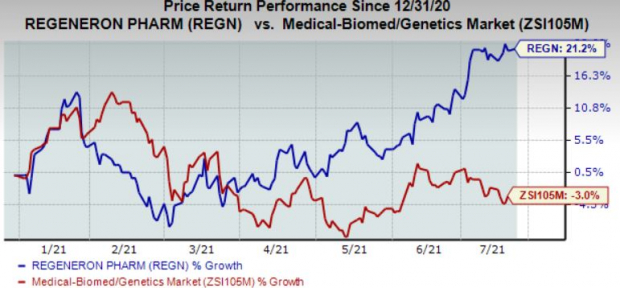
Picture Supply: Zacks Funding Analysis
Regeneron has introduced outcomes from a number of late-stage research demonstrating the flexibility of REGEN-COV to cut back the burden of COVID-19, from prevention to hospitalization.
Regardless of the growing charges of vaccination, the pandemic continues to wreak havoc, giving rise to the necessity for therapies of these contaminated notably in opposition to the variants of concern.
The FDA granted an EUA to GlaxoSmithKline plc GSK and Vir Biotechnology, Inc. VIR ’s sotrovimab, an investigational single-dose monoclonal antibody, for the therapy of mild-to-moderate COVID-19 in adults and pediatric sufferers (12 years of age and older weighing no less than 40 kg).
Regeneron at the moment carries a Zacks Rank #3 (Maintain). You possibly can see the whole record of immediately’s Zacks #1 Rank (Sturdy Purchase) shares right here.
Zacks’ High Picks to Money in on Synthetic Intelligence
In 2021, this world-changing know-how is projected to generate $327.5 billion in income. Now Shark Tank star and billionaire investor Mark Cuban says AI will create “the world’s first trillionaires.” Zacks’ pressing particular report reveals Three AI picks traders have to find out about immediately.
See Three Synthetic Intelligence Shares With Excessive Upside Potential>>
Click on to get this free report
Regeneron Prescribed drugs, Inc. (REGN): Free Inventory Evaluation Report
GlaxoSmithKline plc (GSK): Free Inventory Evaluation Report
Roche Holding AG (RHHBY): Free Inventory Evaluation Report
Vir Biotechnology, Inc. (VIR): Free Inventory Evaluation Report
To learn this text on Zacks.com click on right here.
Zacks Funding Analysis
The views and opinions expressed herein are the views and opinions of the creator and don’t essentially replicate these of Nasdaq, Inc.