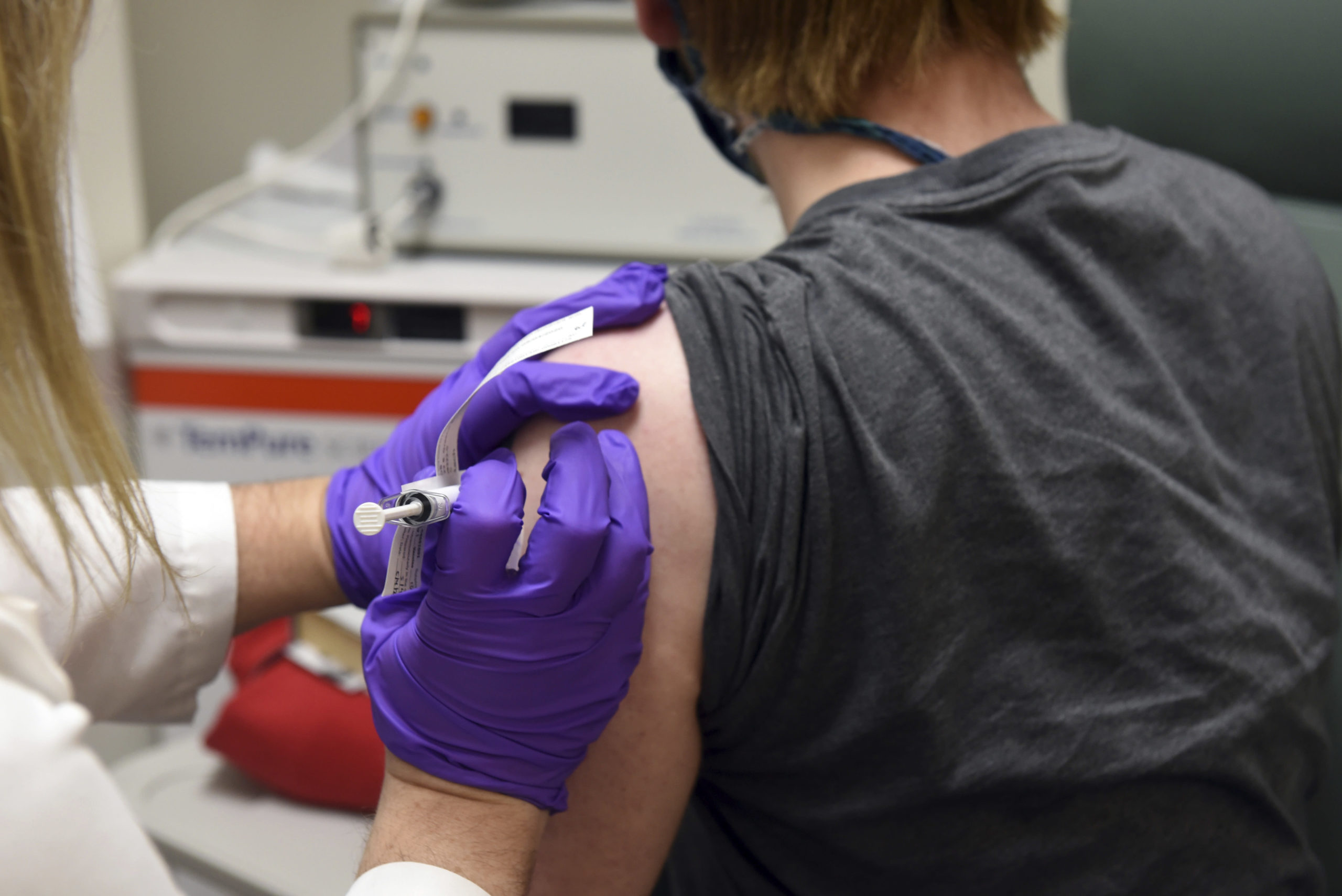
On this Could 4, 2020 photograph supplied by the College of Maryland Faculty of Drugs, the primary affected person enrolled in Pfizer's COVID-19 co
On this Could 4, 2020 photograph supplied by the College of Maryland Faculty of Drugs, the primary affected person enrolled in Pfizer’s COVID-19 coronavirus vaccine scientific trial on the College of Maryland Faculty of Drugs in Baltimore, receives an injection.
College of Maryland Faculty of Drugs | AP
Pfizer may have outcomes from its late-stage coronavirus vaccine trial as early as October, CEO Albert Bourla stated Thursday.
The pharmaceutical firm has already enrolled 23,000 volunteers within the section three trial that started in late July, Bourla stated throughout a Q&A with the Worldwide Federation of Pharmaceutical Producers & Associations, a commerce group. It hopes to enroll at the very least 30,000 members, he stated.
“We anticipate by the top of October, we should always have sufficient … to say whether or not the product works or not,” he stated.
U.S. well being officers have beforehand stated outcomes from late-stage vaccine trials may are available November or sooner.
Pfizer’s potential vaccine is considered one of three backed by the U.S. that is at the moment in late-stage testing. The U.S.-based pharmaceutical large has been working alongside German drugmaker BioNTech. The businesses’ experimental vaccine incorporates genetic materials referred to as messenger RNA, or mRNA. In July, the corporate launched promising knowledge from its early-stage trial.
The section three trial is predicted to incorporate as much as 30,000 members between the ages of 18 and 85 throughout 120 websites globally, together with 39 U.S. states, the corporate has stated. Whether it is profitable, they anticipate to submit it for remaining regulatory overview as early as October. They plan to provide as much as 100 million doses by the top of 2020 and roughly 1.Three billion doses by the top of 2021.
In July, the U.S. authorities introduced it might pay Pfizer and BioNTech $1.95 billion to provide and ship 100 million doses of their vaccine if it proves secure and efficient. The deal was signed as a part of Operation Warp Pace, the Trump administration’s effort to speed up growth and manufacturing of vaccines and coverings to combat the coronavirus.
The CEO’s comment comes because the Facilities for Illness Management and Prevention is asking state governors and native well being departments to organize to distribute a vaccine as quickly as November. The deadline is elevating considerations amongst public well being specialists and scientists that approval of a vaccine can be politically motivated and the White Home could also be pressuring regulators to get a vaccine to the market forward of the presidential election on Nov. 3.
Drug firm executives, together with from Pfizer, have beforehand insisted they are not chopping corners in fast-tracking growth of potential vaccines. They’ve stated the Meals and Drug Administration hasn’t eased its necessities for proving their vaccines are secure and efficient.
Whereas the vaccine could also be secure, the executives have stated it’s “comprehensible” the general public can be involved, including they might want to work to achieve that belief.
“Vaccine hesitancy might be one of many best challenges for public well being that America faces,” John Younger, Pfizer’s chief enterprise officer, instructed Congress on July 21. “All of us must play a task, ought to we achieve success on this mission, that there is confidence within the security and effectiveness of our vaccines primarily based on knowledge, primarily based on confidence the FDA will solely approve a vaccine if it is secure and efficient.”
Bourla stated Thursday that the corporate “would by no means” submit any vaccine for authorization earlier than “we really feel it’s secure and efficient.”
“We won’t minimize corners,” he stated. “Our section three research would be the just one that may enable us to say if we now have a secure and efficient vaccine. If we do not have outcomes from a section three research, we might not submit.”