Shares of Exelixis, Inc. EXEL had been down 23% after it introduced disappointing knowledge from the continuing part III examine, COSMIC-312.
The examine is evaluating Cabometyx together with Roche’s RHHBY Tecentriq (atezolizumab) as in comparison with Nexavar (sorafenib) in sufferers with beforehand untreated superior hepatocellular carcinoma (HCC), a kind of liver most cancers.
Whereas Exelixis is sponsoring COSMIC-312, companion Ipsen is co-funding the trial. Sufferers had been randomized roughly 2:1:1 to one of many three arms — Cabometyx (cabozantinib 40 mg) together with Tecentriq, Nexavar, or Cabometyx (60 mg).
Knowledge from the examine confirmed that it met solely one of many main endpoints, demonstrating important enchancment in progression-free survival (PFS) on the deliberate main evaluation. Cabometyx together with Tecentriq considerably diminished the chance of illness development or demise by 37% as in comparison with Nexavar within the evaluation of the first endpoint of PFS.
Nevertheless, a prespecified interim evaluation for the second main endpoint of general survival (OS) didn’t attain statistical significance.
Therefore, based mostly on these preliminary OS knowledge, Exelixis anticipates the chance of reaching statistical significance on the time of the ultimate evaluation to be low.
However, the examine will proceed as deliberate to the ultimate evaluation of OS and outcomes are anticipated in early 2022. Exelixis additionally plans to debate the trial outcomes and subsequent steps for a possible regulatory submitting with the FDA.
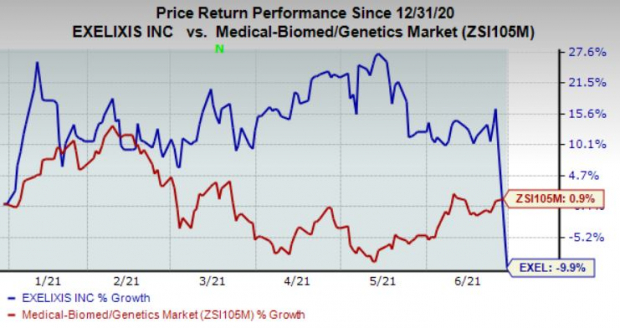
The corporate’s shares have misplaced 9.9% within the yr to this point in contrast with the business’s achieve of 0.9%.
 Picture Supply: Zacks Funding Analysis
Picture Supply: Zacks Funding Analysis
We remind traders that Cabometyx tablets are permitted for the therapy of sufferers with superior renal cell carcinoma (RCC) and HCC, who’ve been beforehand handled with Nexavar.
In January 2021, Exelixis obtained the FDA approval for its supplemental new drug software (sNDA) for Cabometyx together with Bristol Myers’ BMY Opdivo as a first-line therapy of sufferers with superior RCC. Subsequently, in March 2021, Exelixis’ companion Ipsen acquired approval from the European Fee for this mixture as a first-line therapy for superior RCC.
Whereas this market represents big potential, competitors is stiff from the recently-approved mixture therapies.
Exelixis presently carries a Zacks Rank #3 (Maintain). A greater-ranked inventory within the well being care sector is Repligen Corp. RGEN, which presently carries a Zacks Rank #2 (Purchase). You possibly can see the whole record of immediately’s Zacks #1 Rank (Sturdy Purchase) shares right here.
Repligen’s earnings estimates for 2021 have elevated to $2.26 from $1.91 previously 60 days. The inventory worth has elevated 6.6% within the yr to this point.
+1,500% Development: One in every of 2021’s Most Thrilling Funding Alternatives
Along with the shares you examine above, would you prefer to see Zacks’ high picks to capitalize on the Web of Issues (IoT)? It is among the fastest-growing applied sciences in historical past, with an estimated 77 billion gadgets to be related by 2025. That works out to 127 new gadgets per second.
Zacks has launched a particular report that can assist you capitalize on the Web of Issues’s exponential development. It reveals four under-the-radar shares that might be a few of the most worthwhile holdings in your portfolio in 2021 and past.
Click on right here to obtain this report FREE >>
Click on to get this free report
Roche Holding AG (RHHBY): Free Inventory Evaluation Report
Bristol Myers Squibb Firm (BMY): Free Inventory Evaluation Report
Exelixis, Inc. (EXEL): Free Inventory Evaluation Report
Repligen Company (RGEN): Free Inventory Evaluation Report
To learn this text on Zacks.com click on right here.
Zacks Funding Analysis
The views and opinions expressed herein are the views and opinions of the writer and don’t essentially mirror these of Nasdaq, Inc.