QIAGEN N.V. QGEN lately acquire
QIAGEN N.V. QGEN lately acquired FDA approval of its therascreen KRAS RGQ PCR (Polymerase Chain Response) Package for an expanded scope of use. The most recent regulatory go-ahead permits it for use as a companion diagnostic in guiding therapy with Amgen’s AMGN newly-approved lung most cancers focused drug LUMAKRASTM (sotorasib).
QIAGEN has additionally made the speedy launch of this expanded scope of companion diagnostic (CDx) claims for therascreen KRAS Package following the approval. The check might be out there below QIAGEN’s Day One Lab Readiness program.
Extra within the Information
Lumakrastm (sotorasib) is the primary and solely FDA-approved focused therapy for sufferers with KRAS G12C -mutated regionally superior or metastatic non-small cell lung most cancers (NSCLC). It’s a first-in-class drug inhibitor of the G12C-mutated type of the KRAS protein, developed and marketed by Amgen.
QIAGEN’s therascreen tissue CDx KRAS Package claims to detect KRAS G12C, a genetic mutation estimated to be current in as much as 13% of circumstances of NSCLC and normally immune to therapies.
The actual-time qualitative PCR package used with the Rotor-Gene Q MDx instrument is predicted to construct upon QIAGEN’s nine-year expertise in KRAS CDx check growth and commercialization. For traders’ observe, Rotor-Gene Q MDx is a member of QIAGEN’s modular QIAsymphony household of automation options. The launch is predicted to increase the corporate’s therascreen portfolio of companion diagnostic assessments.
The therascreen KRAS Package co-approved with LUMAKRAS was utilized in CodeBreaK 100 scientific trial of sotorasib drug.
Extra Concerning the Launch
To learn sufferers, QIAGEN has accelerated the launch of superior diagnostics by making the therascreen KRAS Package for NSCLC samples available at main laboratories throughout the USA, by means of its Day-One Lab Readiness program for Precision Drugs.
Business Prospects
Per a report printed in Grand View Analysis, the worldwide oncology companion diagnostic market dimension is predicted to see a CAGR of 12.7% from 2020 to 2027. Financial advantages and lowered scientific trial timelines of CDx assays, the FDA’s authorization of simultaneous approval of a diagnostic assay together with its corresponding therapeutic product are among the many key components fueling market progress.
QIAGEN’s superior applied sciences, starting from next-generation sequencing (NGS) to polymerase chain response (PCR), have led it to turn out to be a world chief in companion diagnostic growth. At current, QIAGEN has 10FDA accepted PCR-based companion diagnostic indications and is working with greater than 25 pharmaceutical and biotechnology corporations to develop and commercialize companion diagnostic assessments that advance Precision Drugs.
Notable Developments
Among the many firm’s newest developments, in Could, QIAGEN entered into a world collaboration cope with Mirati Therapeutics Inc. to speed up the event of a tissue-based KRAS companion diagnostic which identifies NSCLC sufferers with KRASG12C mutation, eligible for Mirati’s adagrasib, a selective and potent oral inhibitor of KRAS G12C.
In the identical month, QIAGEN acquired the FDA’s Emergency Use Authorization for QIAreachAnti-SARS-CoV-2 Whole Take a look at, developed in partnership with Australian digital diagnostics firm Ellume. This COVID-19 whole antibody check processes as much as 32 swab samples per hour and supplies easy-to-read outcomes on Ellume’s proprietary eHub in roughly 10 minutes.
In April, the corporate introduced the launch of artus SARS-CoV-2 Prep&Amp UM Package, based mostly on its QIAprep& know-how. This package helps scientific labs strengthen their COVID-19 testing capability with present infrastructure.
In the identical month, the corporate introduced the launch of the LIAISON LymeDetect Assay in collaboration with DiaSorin, to facilitate early detection of Lyme Borreliosis an infection.
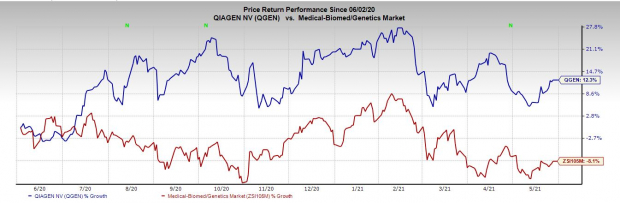
Share Worth Efficiency
The inventory has outperformed its business over the previous yr. It has grown 13.0% towards the business’s decline of 8.0 %.
Zacks Rank and Key Picks
At the moment, QIAGEN carries a Zacks Rank #3 (Maintain).
A couple of better-ranked shares from the broader medical house embody PetIQ, Inc. PETQ, and Nationwide Imaginative and prescient Holdings, Inc. EYE, all sporting a Zacks Rank #1 (Robust Purchase). You’ll be able to see the whole record of in the present day’s Zacks #1 Rank shares right here.
PetIQ has a long-term earnings progress charge of 25.00%.
Nationwide Imaginative and prescient has a long-term earnings progress charge of 23.00%.
Time to Spend money on Authorized Marijuana
In case you’re on the lookout for large positive factors, there couldn’t be a greater time to get in on a younger business primed to skyrocket from $17.7 billion again in 2019 to an anticipated $73.6 billion by 2027.
After a clear sweep of 6 election referendums in 5 states, pot is now authorized in 36 states plus D.C. Federal legalization is predicted quickly and that may very well be a nonetheless higher bonanza for traders. Even earlier than the newest wave of legalization, Zacks Funding Analysis has really helpful pot shares which have shot up as excessive as +285.9%
You’re invited to take a look at Zacks’ Marijuana Moneymakers: An Investor’s Information. It encompasses a well timed Watch Checklist of pot shares and ETFs with distinctive progress potential.
At this time, Obtain Marijuana Moneymakers FREE >>
Click on to get this free report
QIAGEN N.V. (QGEN): Free Inventory Evaluation Report
Amgen Inc. (AMGN): Free Inventory Evaluation Report
PetIQ, Inc. (PETQ): Free Inventory Evaluation Report
Nationwide Imaginative and prescient Holdings, Inc. (EYE): Free Inventory Evaluation Report
To learn this text on Zacks.com click on right here.
Zacks Funding Analysis
The views and opinions expressed herein are the views and opinions of the writer and don’t essentially mirror these of Nasdaq, Inc.