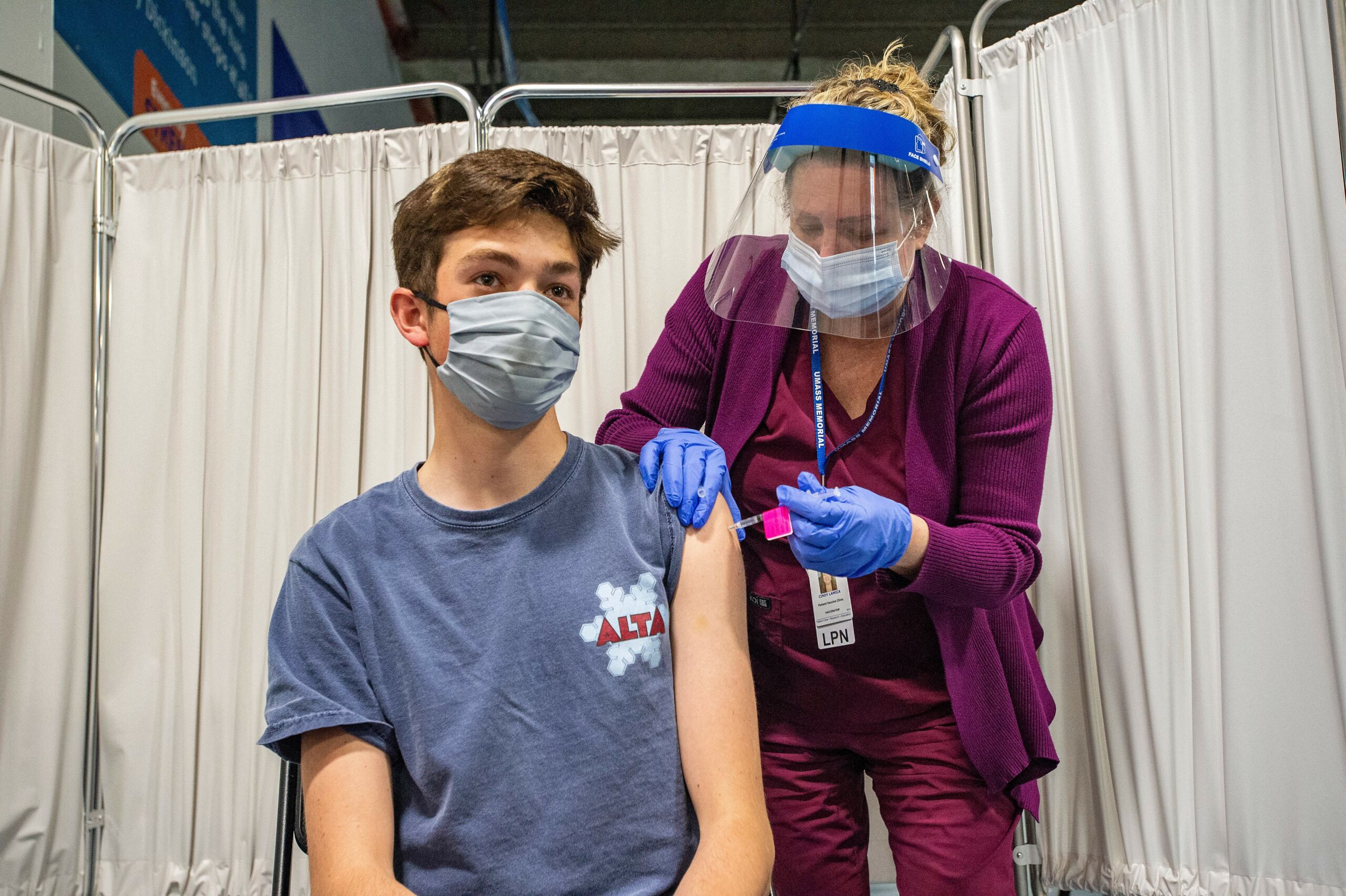
Thomas Gregory, 16, is inoculated by nurse Cindy Lamica with the Pfizer-BioNTech Covid-19 vaccine on the UMass Memorial Well being Care COVID-19 Va
Thomas Gregory, 16, is inoculated by nurse Cindy Lamica with the Pfizer-BioNTech Covid-19 vaccine on the UMass Memorial Well being Care COVID-19 Vaccination Middle within the Mercantile Middle in Worcester, Massachusetts on April 22, 2021.
Joseph Prezioso | AFP | Getty Pictures
Information on how properly the Pfizer-BioNTech Covid-19 vaccine works in children ages 5 to 11 might be obtainable as early as the top of this summer season, the scientist who helped develop the shot advised CNBC.
If scientific trials go properly and the Meals and Drug Administration approves it, younger kids might get vaccinated by the top of the yr, BioNTech o-founder and chief medical officer Dr. Ozlem Tureci mentioned late Thursday.
“We count on the information on the finish of the summer season or autumn of this yr. We are going to then file it with the regulators and, relying on how briskly they react, by the top of the yr we would get approval to additionally immunize youthful kids,” she mentioned.
In late March, Pfizer and BioNTech started a scientific trial testing their vaccine on wholesome 6-month to 11-year-old kids, a vital step in acquiring federal regulatory clearance to start out vaccinating younger children and controlling the pandemic.
For the primary section of the trial, the businesses will determine the popular dosing stage for 3 age teams – between 6 months and a couple of years outdated, 2 and 5, and from ages 5 by 11. The doses shall be evaluated in kids ages 5 by 11 first earlier than researchers transfer on to the opposite age teams, they mentioned.
As a result of the businesses are evaluating the older age group first, it is doable knowledge on children underneath age 5 might come “a bit later,” Tureci advised CNBC.
The 2-dose vaccine is already approved to be used in folks 16 and older. Earlier this month, Pfizer and BioNTech requested the FDA to permit their Covid-19 vaccine to be given to children ages 12 to 15 on an emergency use foundation.
The businesses mentioned in late March that the vaccine was discovered to be 100% efficient in a trial of greater than 2,000 adolescents. In addition they mentioned the vaccine elicited a “sturdy” antibody response within the kids, exceeding these in an earlier trial of older teenagers and younger adults. Unwanted effects have been usually in line with these seen in adults, they added.
Vaccinating kids is seen as essential to ending the pandemic. The nation is unlikely to attain herd immunity — when sufficient folks in a given neighborhood have antibodies in opposition to a selected illness — till kids can get vaccinated, well being officers and consultants say.
Youngsters make up round 20% of the entire U.S. inhabitants, in response to authorities knowledge. Between 70% and 85% of the U.S. inhabitants must be vaccinated in opposition to Covid to attain herd immunity, consultants say, and a few adults might refuse to get the pictures.
Along with testing the vaccine in younger kids, Pfizer and BioNTech are testing whether or not a 3rd dose of the vaccine would offer a greater immune response in opposition to new variants of the virus.
BioNTech CEO Ugur Sahin advised CNBC on Thursday he’s “assured” the vaccine is efficient in opposition to B.1.617, a extremely contagious coronavirus variant first recognized in India.
Nonetheless, he mentioned, folks will probably want a 3rd shot of its two-dose vaccine as immunity in opposition to the virus wanes. Researchers are seeing a decline in antibody responses in opposition to the virus after eight months, he added.
“If we offer a lift we might actually amplify the antibody response even above the degrees that we had in the beginning and that would give us actual consolation for cover for not less than 12 months, perhaps 18 months,” Sahin mentioned. “And that is actually vital in a time the place all of the variants are coming in.”
Sahin additionally mentioned he expects demand for the shot to proceed to extend, including the corporate is boosting the manufacturing capability of the vaccine to three billion doses by the top of 2021. In December, Sahin expects the corporate’s manufacturing purpose will go as much as 400 million doses a month.