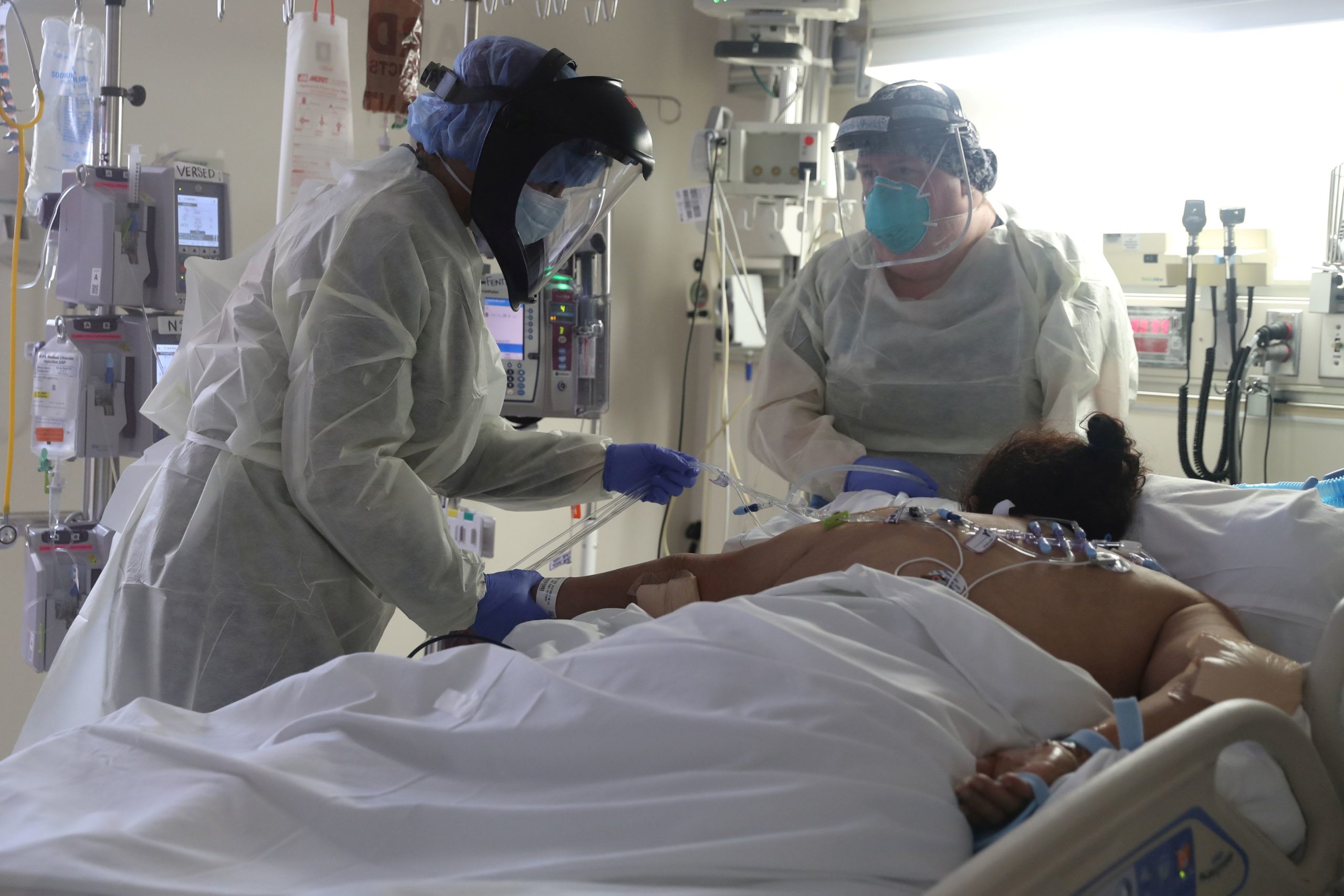
Medical employees attend to a affected person affected by the coronavirus illness (COVID-19) within the Intensive Care Unit (ICU), at Scripps Mercy
Medical employees attend to a affected person affected by the coronavirus illness (COVID-19) within the Intensive Care Unit (ICU), at Scripps Mercy Hospital in Chula Vista, California, U.S., Might 12, 2020.
Lucy Nicholson | Reuters
The Meals and Drug Administration has accepted the emergency use of Abiomed’s Impella coronary heart pump to assist deal with Covid-19 sufferers affected by coronary heart and lung failure, the corporate introduced Tuesday.
In 1 in 10 sufferers, the virus ends in excessive irritation of the guts, along with a build-up of fluid within the lungs. For 42-year-old Devan Smith, who had no historical past of coronary heart illness, the mixture proved almost deadly in Might.
“This wasn’t a coronary heart assault within the conventional sense. It was a pure case of myocarditis,” or irritation of the guts muscle, mentioned Dr. John Finley, a heart specialist at Mercy Catholic Medical Heart exterior Philadelphia. “No prior to we might get him again to the ICU, that he arrested in all probability 10 to 15 occasions.”
Medical doctors at Mercy had first put Smith on a ventilator, however when his coronary heart started to fail they determined to attempt utilizing Abiomed’s Impella coronary heart pump to attempt to give his coronary heart muscle a relaxation, together with an extracorporeal membrane oxygenation machine to pump extra oxygen into his bloodstream. It is a twin therapy usually used for high-risk cardiac sufferers.
After 5 days, Smith was in a position to come off the machines and inside a number of weeks he had totally recovered.
“It makes you take a look at life and respect life a lot better now, figuring out that … two months in the past, principally I’d have died,” mentioned Smith, who lately returned to his job as a warehouse employee in Philadelphia.
The Impella shouldn’t be a brand new machine, nevertheless it’s the primary time it has obtained FDA approval of the emergency use for treating the guts together with an ECMO for coronavirus sufferers.
“It has been round for some time as a result of individuals have seen that if a affected person has to go on ECMO, generally the ECMO places an excessive amount of of a load on the guts,” mentioned Dr. Charles Simonton, Abiomed chief medical officer, “nevertheless it does have a singular utility for Covid, since there is not any different possibility like this for sufferers.”
Earlier this summer season, the FDA approved the emergency use of Abiomed’s Impella as a standalone therapy to stabilize Covid sufferers following the removing of pulmonary blood clots.
Smith was considered one of three sufferers who obtained the mixture therapy in Pennsylvania this spring, and all three sufferers recovered. Whereas Covid-19 sufferers typically undergo persevering with well being points after restoration, in Smith’s case there are not any obvious bodily after-effects.
“His coronary heart is regular, which is unimaginable. They even did a cardiac MRI to search for a scar or residual irritation and it was regular,” as had been his lungs and kidneys, mentioned Finley.
“They saved my life!” Smith mentioned. “1,000,000 occasions I say thanks!”